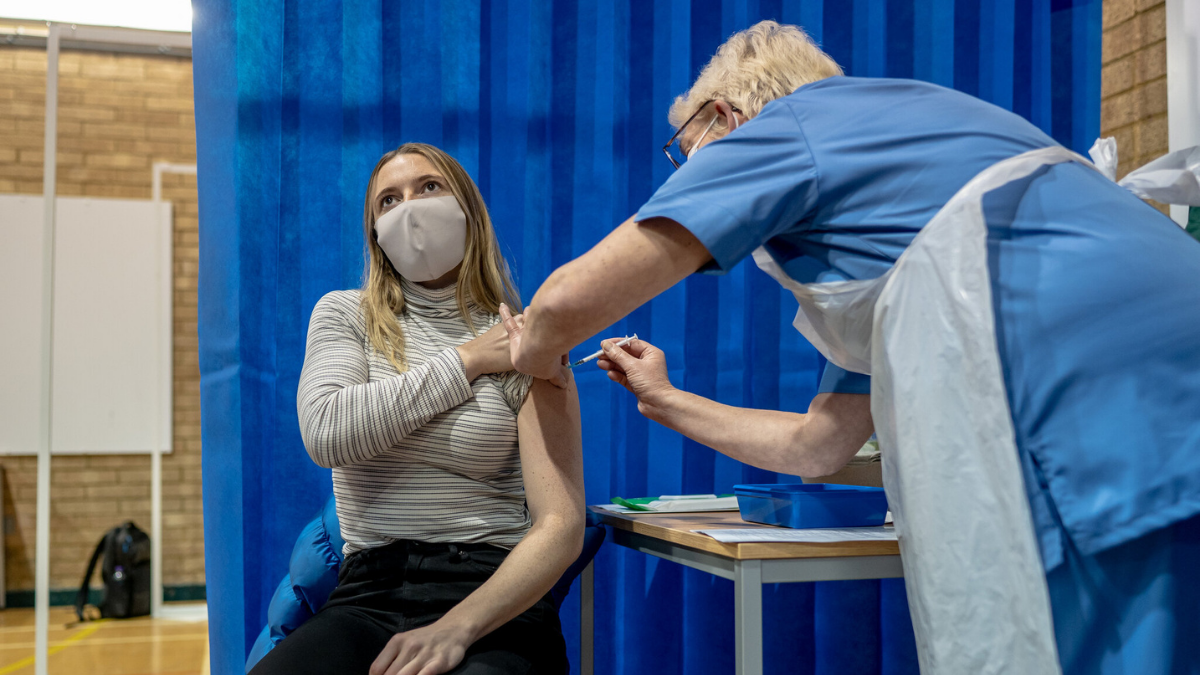
LONDON – U.K. health regulators have warned people with a “significant history’’ of allergic reactions shouldn’t receive the new coronavirus vaccine while they investigate two recipients who had reactions.
The U.K. began rolling out the vaccine on Tuesday after getting approval on December 2. Professor Stephen Powis, national medical director for the National Health Service in England said two healthcare workers with a history of severe allergies reacted to the vaccine.
“As is common with new vaccines, the MHRA has advised, on a precautionary basis, that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday,’’ Powis said in a statement. “Both are recovering well.”
Dr. June Raine, head of the U.K.’s medical regulatory agency, said the reactions are being monitored and her team will be closely monitoring those who receive the Pfizer vaccine. It is not yet known the element of the vaccine the staff members were allergic to.
“The role is before, during and after, and there is a true end-to-end looking from the scientific laboratory bench through to the patient who yesterday first received the vaccine,” she said.
Beginning Wednesday, all patients who are set to get the vaccine will be asked beforehand if they have a history of allergic reactions.
A Pfizer spokesperson told Sky News its vaccine was “well tolerated” during trials and there were “no serious safety concerns.”
“We have been advised by MHRA of two yellow card reports that may be associated with allergic reaction due to administration of the COVID-19 BNT162b2 vaccine,” a spokesperson for the company said.
“As a precautionary measure, the MHRA has issued temporary guidance to the NHS while it conducts an investigation in order to fully understand each case and its causes. Pfizer and BioNTech are supporting the MHRA in the investigation.”
The United States has not approved the vaccine yet but could do so as early as Thursday.